Coral-shaped PtRu/Ni(OH)2 electrocatalyst promotes selective hydrogenation of benzoic acid†
Abstract
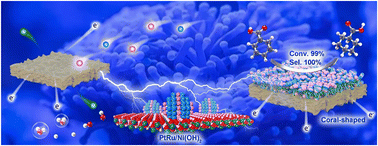
A series of 3D self-standing PtRu/Ni(OH)2/NF electrodes is fabricated for electrocatalytic hydrogenation (ECH) of benzoic acid (BA) to cyclohexanecarboxylic acid (CCA). The PtRu/Ni(OH)2 electrocatalyst with a unique coral-shaped architecture possesses outstanding charge transfer ability and abundant active sites. The optimized electrode achieves a BA conversion of 99.00% and a CCA selectivity of 100% in 0.05 M H2SO4 within 80 min, showing a record faradaic efficiency of 43.90% and high performance during 20 cycles of ECH. In situ Raman tests verify the mechanism of the selective hydrogenation of the aromatic ring and density functional theory (DFT) calculations reveal that the electronic interaction between Pt and Ru in the PtRu nanoalloy as well as the electron transfer from the PtRu alloy to the Ni(OH)2 nanosheet tunes the electronic states of PtRu/Ni(OH)2, thereby enhancing the adsorption strength of BA on the electrocatalyst surface. This work offers a promising alternative to the traditional catalytic hydrogenation of BA.


 Please wait while we load your content...
Please wait while we load your content...