The effect of the C5-substituent on regioselectivity in the Rh(i)-catalyzed intermolecular transannulation of 1,2,3-thiadiazoles with phenylacetylene†
Abstract
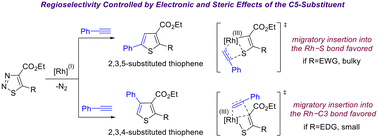
Despite the growing use of denitrogenative reactions of 1,2,3-thiadiazoles in heterocycle synthesis, the origin of the varied reactivity and divergent regioselectivity observed in their transannulation reactions is not well understood. To address this limitation, systematic studies on the reactivity and regioselectivity in the Rh(I)-catalyzed intermolecular transannulation between a range of substituted 1,2,3-thiadiazoles and phenylacetylene have been conducted. Our experimental data revealed that the electronic nature of the C5-substituent on the 1,2,3-thiadiazole ring influenced reactivity significantly. Moreover, the nature of the substituent was shown to have a remarkable effect on the regioselectivity of the reaction, with electron-donating groups leading to 2,3,4-substituted thiophenes and strong electron-withdrawing groups favouring the 2,3,5-substituted products. Identified experimental trends have been supported and rationalized using density functional theory calculations.


 Please wait while we load your content...
Please wait while we load your content...