Water-assisted sonochemically-induced demethylenation of benzyl alcohol to phenol over a structurally stable cupric oxide catalyst†
Abstract
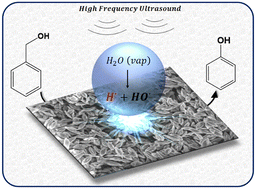
Novel catalytic chemistry of demethylenation of benzyl alcohol to phenol is presented here using the synergy between an earth-abundant transition metal oxide (CuO) catalyst and high frequency ultrasound (HFUS). This chemistry is achieved in water and at room temperature. Using a combination of catalyst characterization, chemical and acoustic analysis, isotope labelling and density functional theory computations, we reveal the molecular reaction mechanism, involving benzaldehyde as an intermediate. Water is not just a benign solvation medium, but it directly participates in the chemistry by getting dissociated due to sonolysis. The adsorption of the OH from water on the catalyst surface inhibits its recombination. The surface adsorbed OH from water also activates the C–H bond in benzyl alcohol to form benzaldehyde and later incorporates itself into the phenyl ring to form phenol.


 Please wait while we load your content...
Please wait while we load your content...