Electrocatalytic hydrogen evolution by robust square planar nickel complexes in an S2P2 coordination environment†
Abstract
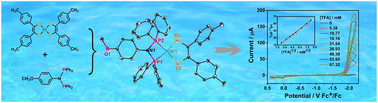
Stimulated by the structural feature of the [NiFe]-hydrogenase active sites, five new heteroleptic 1,2-bis(p-substitutedphenyl)ethylene-1,2-dithiolate nickel complexes containing the N-(p-methoxyphenyl)-bis(diphenylphosphine)amine ligand, [p-CH3OC6H4N(PPh2)2]Ni[S2C2(C6H4R-p)2)] (R = CH3O (1), CH3 (2), H (3), Br (4), and F (5)), were synthesized and characterized. The crystal structure analysis of 2 and 4·CH2Cl2 indicates that the two complexes have an almost perfect S2P2 square planar coordination environment defined by the 1,2-diarylethylene-1,2-dithiolate and bis(diphenylphosphine)amine ligands. The electrochemical behavior and electrocatalytic hydrogen-evolving activity were investigated using trifluoroacetic acid (TFA) as the proton source in combination with cyclic voltammetry (CV) and controlled potential electrolysis (CPE). The turnover frequencies (TOF) and overpotentials (η) for hydrogen evolution of 1–5 (0.25 mM) in DMF are estimated to be 423–894 s−1 and 0.91–1.02 V with 67.32 mM TFA, respectively. Faradaic efficiencies (FE) of 66.15–98.20% and turnover numbers (TON) of 13.16–35.92 are achieved under the CPE experiments over 18 hour periods at −2.00 V vs. Fc+/Fc, respectively. Density functional theory (DFT) calculations display that the electron-withdrawing substituent on the 1,2-diarylethylene-1,2-dithiolate ligand can reduce the energy levels of the highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) of the complex, leading to lower negative redox and catalytic potentials. On the basis of the experiments and the theoretical calculations, an ECEC electrocatalytic mechanism for hydrogen production is proposed. Intriguingly, excellent electrochemical stability of 1–5 in hydrogen generation is observed, which can act as potential candidates for electrocatalysts with high tolerance. These research findings confirm that the synthesized heteroleptic complexes can serve as homogeneous molecular electrocatalysts for hydrogen production with high tolerance.


 Please wait while we load your content...
Please wait while we load your content...