Stereo-controlled cyclopropanation catalysis within the confined pores of porphyrin MOFs†
Abstract
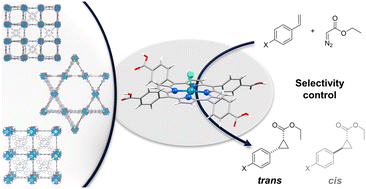
In analogy to the confined reaction environment in enzymes where different functionalities are responsible for substrate coordination, specific substrate–catalyst interactions within the pores of metal–organic frameworks (MOFs) can impact the catalytic selectivity. We applied topologically different, Rh-metalated porphyrin-based MOFs as nanoreactors in the cyclopropanation of styrene and its derivatives as a model reaction to examine reaction space controlled stereoselectivity differences. The porphyrin-based MOF scaffold leads to a clear diastereoselectivity enhancement, exclusively yielding the trans isomer, which is not observed using a structurally analogous molecular catalyst operating in homogeneous phase. In order to elucidate the origin of the unprecedented diastereoselectivity using MOF catalysts we have studied the effect of donor additives, namely pyridine, aniline, ethylene diamine and phenol, on the activity and selectivity – increasing the activity of the reaction by decreasing the energy barrier for Rh-carbene formation without affecting the selectivity which makes the possibility of previously described anchoring to neighboring Rh sites unlikely. The MOF topology independent reactivity observations suggest a coordination of the substrate to the MOF framework as cause for the enhanced diastereoselectivity. This allows for the extraction of conceptual principles for porous catalyst design.


 Please wait while we load your content...
Please wait while we load your content...