Covalent organic frameworks editing for efficient metallaphotoredox catalytic carbon–oxygen cross coupling of aryl halides with alcohols†
Abstract
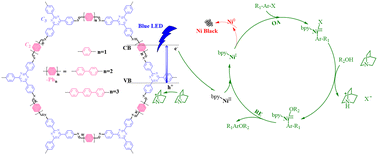
Cross-coupling by dual metal/photoredox catalysis is attractive for producing valuable chemical building blocks, where the photoredox catalysts lay the foundations for an efficient and sustainable operation. Herein a series of imine based hexagonal covalent organic frameworks (COFs) were developed as photoredox platforms to cooperate with a molecular Ni catalyst for the cross-coupling of aryl halides with alcohols. By varying the C2 and/or C3 symmetric molecular subunits, the catalytic performance was effectivity modulated with up to one magnitude difference in the solar-to-coupling product conversion efficiency. Combined (photo)electrochemical measurements and electron paramagnetic resonance spectroscopy revealed the essence of Ni speciation balance to sustain the level of active Ni(I) and the productive coupling cycle. Specifically, 4-[4-(4-formylphenyl)phenyl]benzaldehyde and planar 4,4′,4′′-(1,3,5-triazine-2,4,6-triyl)trianiline, upon assembling into TT–Ph3 COF, translated to band structures that ensure Ni(II) reduction by conduction band electrons to Ni(I) and minimal off-pathway deactivation, granting the best cross-coupling performance.


 Please wait while we load your content...
Please wait while we load your content...