Charged species redistribution at electrochemical interfaces: a model system of the zirconium oxide/water interface†
Abstract
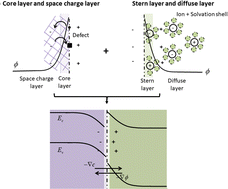
Quantifying the local distribution of charged defects in the solid state and charged ions in liquid solution near the oxide/liquid interface is key to understanding a range of important electrochemical processes, including oxygen reduction and evolution, corrosion and hydrogen evolution reactions. Based on a grand canonical approach relying on the electrochemical potential of individual charged species, a unified treatment of charged defects on the solid side and ions on the water side can be established. This approach is compatible with first-principles calculations where the formation free energy of individual charged species can be calculated and modulated by imposing certain electrochemical potential. Herein, we apply this framework to a system of monoclinic ZrO2(![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 11)/water interface. The structure, defect chemistry and dynamical behavior of the electric double layer and space charge layer are analyzed with different pH values, water chemistry and doping elements in zirconium oxide. The model predicts ZrO2 solubility in water and the point of zero charge consistent with the experimentally-measured values. We reveal the effect of dopant elements on the concentrations of oxygen and hydrogen species at the surface of the ZrO2 passive layer in contact with water, uncovering an intrinsic trade-off between oxygen diffusion and hydrogen pickup during the corrosion of zirconium alloys. The solid/water interface model established here serves as the basis for modeling reaction and transport kinetics under doping and water chemistry effects.
11)/water interface. The structure, defect chemistry and dynamical behavior of the electric double layer and space charge layer are analyzed with different pH values, water chemistry and doping elements in zirconium oxide. The model predicts ZrO2 solubility in water and the point of zero charge consistent with the experimentally-measured values. We reveal the effect of dopant elements on the concentrations of oxygen and hydrogen species at the surface of the ZrO2 passive layer in contact with water, uncovering an intrinsic trade-off between oxygen diffusion and hydrogen pickup during the corrosion of zirconium alloys. The solid/water interface model established here serves as the basis for modeling reaction and transport kinetics under doping and water chemistry effects.


 Please wait while we load your content...
Please wait while we load your content...