Adsorption properties of small gas molecules on SnSe2 monolayer supported with transition metal: first-principles calculations†
Abstract
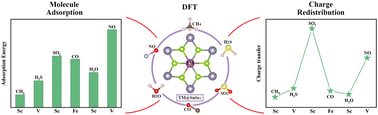
The adsorption properties of CH4, H2S, SO2, CO, H2O and NO molecules on transition metal-supported SnSe2 surface are investigated by the first-principles method. The calculation results show that the transition metal (TM = Sc, Ti, V, Cr, Mn, Fe, Co, Ni, Cu) has the lowest adsorption energy when supporting at the Sn site of SnSe2, indicating the system is relatively stable. Also, we find that CH4, SO2 and H2O molecules tend to adsorb on Sc-supported SnSe2 surface, H2S and NO molecules prefer to adsorb on V-supported SnSe2 surface, while CO molecule and Fe-supported SnSe2 surfaces have strong interaction. And, CH4, H2S and H2O molecules act as donors to provide electrons to the substrate, while SO2, CO and NO molecules act as acceptors to gain electrons from the substrate. An analysis of charge difference density and density of states reveals that the adsorption energies of gas molecules are related to charge transfer and orbital hybridization. We hope that this work not only provides a promising sensor material, but also provides a new idea for the rational design of two-dimensional materials.
- This article is part of the themed collection: 2023 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...