Influence of non-covalent interactions on the coordination geometry of Ni(ii) in Ni(ii)–M(ii) complexes (M = Zn and Hg) with a salen-type N2O2 Schiff base ligand and thiocyanate ion as the coligand†
Abstract
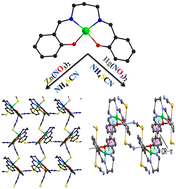
A nickel(II)–zinc(II) complex, [(NiL)Zn(μ1,3-NCS)2]n (1), and a nickel(II)–mercury(II) complex, [{(NiL)Hg(μ1,3-SCN)(SCN)}2] (2), (where H2L = N,N′-bis(salicylidene)-1,3-propanediamine) were synthesized via a metalloligand approach, in which the former is a 2D coordination polymer and the latter is a discrete tetramer. Single-crystal X-ray crystallography revealed that Zn(II) in 1 and Hg(II) in 2 are in a four-coordinated distorted tetrahedral environment whereas the geometry of the Ni(II) centre is distorted octahedral for complex 1 but distorted square planar for complex 2. DFT calculations were used to analyze an intramolecular weak interaction between the square planar Ni(II) ion and the N-end of thiocyanate ions, which can be defined as a π-hole interaction. Moreover, we analyzed the tetranuclear structure of 2 in terms of a self-assembled dimer via the formation of two strong spodium bonds instead of Hg–N coordination bonds, as evidenced by the QTAIM and NCI plot analysis and the magnitude of the dimerization energy.


 Please wait while we load your content...
Please wait while we load your content...