Novel salts and cocrystals of the antifolate drug trimethoprim and their role in the enhancement of solubility and dissolution†
Abstract
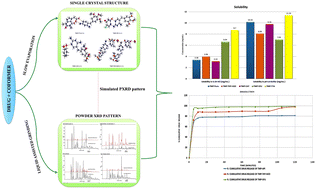
Trimethoprim (TMP) is a BCS class II anti-folate drug with poor aqueous solubility. Four new crystal structures of TMP were developed in this work using a supramolecular synthon approach with coformers theophylline-7-acetic acid (T7A), 5-fluorouracil (5FU), catechol (CAT) and thymine (THY). The carboxylate or amide or hydroxyl groups present on coformer structures aided in generating heterosynthons with the aminopyrimidine ring of TMP. TMP–T7A and TMP–5FU existed as salts, whereas TMP–CAT and TMP–THY–H2O formed as cocrystals. The crystal packing, melting behavior, solubility and dissolution profiles of all four systems were investigated in this study. The cocrystal hydrate (TMP–THY–H2O) displayed lowest melting and TMP–T7A salt displayed the highest melting temperature. Various factors, including the nature of coformers (acidic/basic/ionizable/non-ionizable), pH and pKa, altered the aqueous solubility of TMP. Among the four systems, TMP–T7A, which possesses the aminopyrimidinium-carboxylate synthon, showed highest solubility compared with the structures containing aminopyrimidinium-amide/hydroxyl synthons.


 Please wait while we load your content...
Please wait while we load your content...