Cage-like structures based on constrained cyclic arylopeptoids†
Abstract
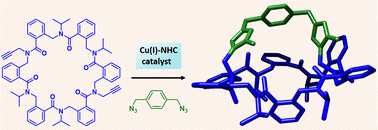
The access to cupola-like or tube-like structures from ortho- and meta-arylopeptoid macrocycles was explored through CuAAC reaction using a partially flexible bis(azide) and CuI-N-heterocyclic carbene as catalyst. NMR studies showed that a bis-triazolium bicylic compound in the ortho-series adopts well-defined structure in polar aprotic and protic solvents. Besides, preliminary study revealed its potential for oxoanion recognition.


 Please wait while we load your content...
Please wait while we load your content...