Rh(iii)-catalyzed regioselective versatile indole derivatization: delivering potential of rare β-(1H-indol-2-yl)-β-amino acids in one pot†
Abstract
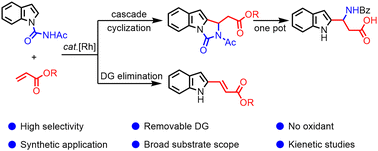
Rh-catalyzed selective C–H functionalization of indoles with two routes was developed: an alkenylation–annulation with the addition of KHSO4 and alkenylation–elimination in the presence of CsOAc to the corresponding products, respectively. Notably, one-pot hydrolysis and benzoylation of the annulation products successfully afforded easily separable β-(1H-indol-2-yl)-β-amino acid derivatives.


 Please wait while we load your content...
Please wait while we load your content...