Synthesis and antitumor activities of α-hydroxyamino phosphine oxides by catalyst-free hydrophosphinylation of nitrones†
Abstract
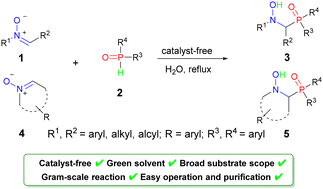
Inspired by the diverse bioactivities of α-amino phosphine oxides, an efficient strategy for the synthesis of less researched α-(hydroxyamino)diarylphosphine oxides has been developed and their antitumor activities are explored. Under water as a solvent and catalyst-free conditions, the addition of nitrones and diphenylphosphine oxide occurs smoothly to afford α-(hydroxyamino) diarylphosphine oxides in high yields. This reaction features a wide substrate scope, facile starting materials, atom economy, and easy purification. Moreover, the biological evaluation revealed that two synthesized derivatives 5e and 5f could serve as interesting anti-cancer agents for further development.


 Please wait while we load your content...
Please wait while we load your content...