Beryllium-centred C–H activation of benzene†
Abstract
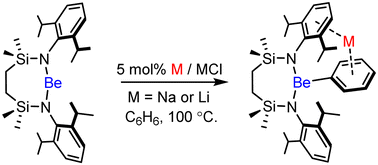
Reaction of BeCl2 with the dilithium diamide, [{SiNDipp}Li2] ({SiNDipp} = {CH2SiMe2NDipp}2), provides the dimeric chloroberyllate, [{SiNDippBeCl}Li]2, en route to the 2-coordinate beryllium amide, [SiNDippBe]. Lithium or sodium reduction of [SiNDippBe] in benzene, provides the relevant organoberyllate products, [{SiNDippBePh}M] (M = Li or Na), via the presumed intermediacy of transient Be(I) radicals.
- This article is part of the themed collection: Chemical Communications HOT Articles 2023


 Please wait while we load your content...
Please wait while we load your content...