Intimate relationship between C–I reductive elimination, aryl scrambling and isomerization processes in Au(iii) complexes†
Abstract
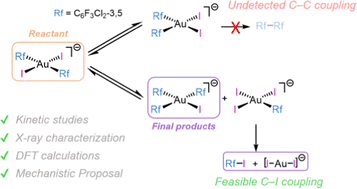
19F NMR monitoring shows that heating trans-[AuIIIRf2I2]− solutions (Rf = C6F3Cl2-3,5) leads to formation of cis-[AuRf2I2]−, [AuRf3I]− and [AuRfI3]−via kinetic competition between isomerization and Rf/I scrambling. The system evolution is driven by the easy Rf–I reductive elimination from [AuRfI3]− (forming also [AuI2]−), which is faster than any of the Rf–Rf couplings from the coexisting species, hindering the commonly desired and thermodynamically preferred C–C coupling. A kinetic model where I− dissociation triggers both isomerization and transmetalation steps is proposed, which fits well the experimental data. DFT calculations support that the lower bond strength of AuIII–I compared to other halides produces a pathway switch that makes C–I coupling kinetically preferred. Consequently, it is better avoided in reactions looking for C–C coupling.
- This article is part of the themed collection: Chemical Communications HOT Articles 2023


 Please wait while we load your content...
Please wait while we load your content...