Reactivity of quinone methides with carbenes generated from α-diazocarbonyl compounds and related compounds
Abstract
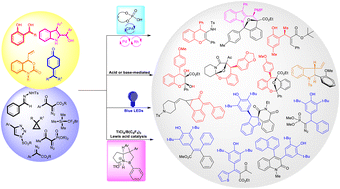
Over the years, quinone methides have broadly been applied in synthesis and biological systems for synthesizing heterocyclic compounds and biologically active molecules. In this feature article, we have discussed the novel and uncovered reactivity of o-quinone methides, p-quinone methides, aza-o-quinone methides, and indolyl-2-methides with carbenes generated from α-diazocarbonyl compounds and related compounds. Two in situ-generated transient intermediates undergo cycloannulation reactions, metathesis-type reactions, 1,6-conjugate addition reactions, cyclopropanation reactions, and many other transformations to access nitrogen- and oxygen-containing heterocyclic compounds and beyond. The reactivity of quinone methides and carbenes is observed in various metal catalysts, Brønsted-acids, Lewis acids, phase transfer catalysts, additives, and visible-light-induced transformations.


 Please wait while we load your content...
Please wait while we load your content...