Antiferromagnetic quaternary chalco-halide Ba3(FeS4)I with long Fe⋯Fe distances†
Abstract
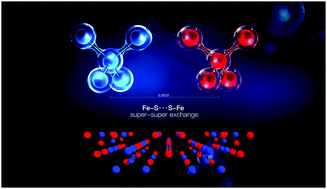
A novel quaternary chalco-halide Ba3(FeS4)I was successfully synthesized via a molten salt method. The Ba3(FeS4)I crystallizes in the orthorhombic space group Cmcm (NO. 63). It features two dimensional [Ba3I]5+ layers, which are separated by isolated [FeS4]5− tetrahedra. Ba3(FeS4)I is a semiconductor with a direct band gap of 1.65 eV. Despite the large Fe⋯Fe separation (≥6.293 Å), the Ba3(FeS4)I shows unexpected antiferromagnetic ordering with Néel temperatures (TN) of 108 K. The possible magnetic structures and the underlying magnetic interaction mechanism were proposed based on the first-principles electronic structure calculations. The [FeS4]5− tetrahedra behave like super-moments due to the strong p–d hybridization between Fe and S, making the S atoms adopt the same spin orientation to their nearest-neighboring Fe atoms. The new magnetic interaction mechanism, namely the Fe–S⋯S–Fe super–super exchange, was found to be responsible for this antiferromagnetic phase transition.


 Please wait while we load your content...
Please wait while we load your content...