A novel decellularized trachea preparation method for the rapid construction of a functional tissue engineered trachea to repair tracheal defects
Abstract
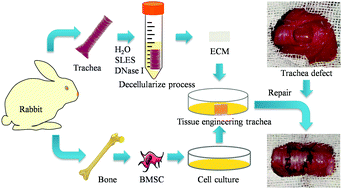
Long segment trachea defects are repaired by tracheal substitution, while decellularized technology has been effectively employed to prepare tissue engineering trachea (TET). However, its clinical application is restricted by the long preparation cycle, while poor vascularization is associated with the transplantation failure. In the present study, we used sodium lauryl ether sulfate (SLES) to develop a novel rapid decellularized tracheal preparation method, then constructed a TET with revascularization functions. Summarily, we decellularized rabbit trachea using various SLES concentrations. Results from histological analysis, immunohistochemical and DAPI staining, as well as DNA quantitative assay, revealed that 1–0.1% (v/v) SLES treatment not only entirely removed cellular components to reduce its immunogenicity, but also retained the tracheal matrix's gross structure. SEM images, safranine O-fast green staining, total collagen content assay and collagen II immunofluorescence revealed that low SLES concentrations preserved the bioactive components of the decellularized tracheal matrix. Next, we performed cytobiocompatible and cytotoxin assays to verify biocompatibility of the decellularized tracheal matrix, and is confirmed by the omentum transplantation of rats. Results from omentum transplantation revealed that the decellularized tracheal matrix had low immunogenicity and excellent biocompatibility. Its revascularization capacity was confirmed by histologic appearance and CD31 immunofluorescence. Based on these findings, we selected 0.1% (v/v) as the optimal SLES concentration for preparing a decellularized tracheal matrix. Next, we seeded allogeneic bone marrow stem cells (BMSC) onto the matrix to construct TET patches. In vivo tracheal defect reconstruction confirmed the biocompatibility and revascularization capacity of this novel TET, and the formation of a vascular network around the patch promoted submucosa and mucosa regeneration without significant stenosis, 4 weeks post-surgery. In conclusion, we used SLES to successfully develop a novel decellularized approach for the preparation of TET, which has low immunogenic and inflammatory responses, as well as excellent biocompatibility, and revascularization ability in vivo without additional exogenous cytokines.


 Please wait while we load your content...
Please wait while we load your content...