In vivo visualization of enantioselective targeting of amyloid and improvement of cognitive function by clickable chiral metallohelices†
Abstract
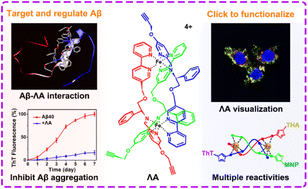
The pathogenesis of Alzheimer's disease (AD) is closely related to several contributing factors, especially amyloid-β (Aβ) aggregation. Bioorthogonal reactions provide a general, facile, and robust route for the localization and derivatization of Aβ-targeted agents. Herein, a pair of chiral alkyne-containing metallohelices (ΛA and ΔA) were demonstrated to enantioselectively target and modulate Aβ aggregation, which has been monitored in triple-transgenic AD model mice and proved to improve cognitive function. Compared with its enantiomer ΔA, ΛA performed better in blocking Aβ fibrillation, relieving Aβ-triggered toxicity, and recovering memory deficits in vivo. Moreover, clickable ΛA could act as a functional module for subsequent visualization and versatile modification of amyloid via bioorthogonal reaction. As a proof-of-concept, thioflavin T, tacrine, and magnetic nanoparticles were conjugated with ΛA to realize Aβ photo-oxygenation, acetylcholinesterase inhibition, and Aβ clearance, respectively. This proof-of-principle work provided new insights into the biolabeling and bioconjugation of multifunctional metallosupramolecules through click reactions for AD therapy.
- This article is part of the themed collection: 2023 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...