Turn-on fluorescent capsule for selective fluoride detection and water purification†
Abstract
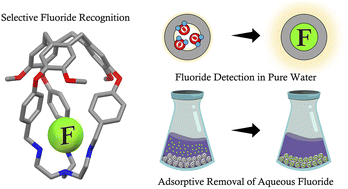
It has been a long-standing challenge to develop organic molecular capsules for selective anion binding in water. Here, selective recognition of aqueous fluoride was achieved through triple protonation of a hemicryptophane (L), which is composed of a fluorescent cyclotriveratrylene (CTV) cap and tris(2-aminoethyl)amine (tren) as the anion binding site. Fluoride encapsulation by [3H-L]3+ was evidenced by 1H NMR, 19F NMR, LC-MS, and X-ray crystallography. In addition, [3H-L]3+ exhibited a ‘turn-on’ fluorescence signal (λem = 324 nm) upon fluoride addition. An apparent association constant KA = (7.5 ± 0.4) × 104 M−1 and a detection limit of 570 nM fluoride were extracted from the fluorescence titration experiments in citrate buffer at pH 4.1. To the best of our knowledge, [3H-L]3+ is the first example of a metal-free molecular capsule that reports on fluoride binding in purely aqueous solutions with a fluorescence response. Finally, the protonated capsule was supported on silica gel, which enabled adsorptive removal of stoichiometric fluoride from water and highlights real-world applications of this organic host–guest chemistry.


 Please wait while we load your content...
Please wait while we load your content...