Rhodium-catalyzed enantioselective C–H alkynylation of sulfoxides in diverse patterns: desymmetrization, kinetic resolution, and parallel kinetic resolution†
Abstract
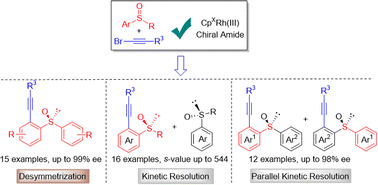
Rhodium-catalyzed enantioselective C–H alkynylation of achiral and racemic sulfoxides is disclosed with alkynyl bromide as the alkynylating reagent. A wide range of chiral sulfoxides have been constructed in good yield and excellent enantioselectivity (up to 99% ee, s-factor up to > 500) via desymmetrization, kinetic resolution, and parallel kinetic resolution under mild reaction conditions. The high enantioselectivity was rendered by the chiral cyclopentadienyl rhodium(III) catalyst paired with a chiral carboxamide additive. The interactions between the chiral catalyst, the sulfoxide, and the chiral carboxylic amide during the C–H bond cleavage offer the asymmetric induction, which is validated by DFT calculations. The chiral carboxamide functions as a base to promote C–H activation and offers an additional chiral environment during the C–H cleavage.


 Please wait while we load your content...
Please wait while we load your content...