Synergistic effects of CH3CO2H and Ca2+ on C–H bond activation by MnO4−†
Abstract
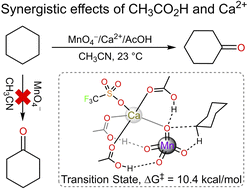
The activation of metal-oxo species with Lewis acids is of current interest. In this work, the effects of a weak Brønsted acid such as CH3CO2H and a weak Lewis acid such as Ca2+ on C–H bond activation by KMnO4 have been investigated. Although MnO4− is rather non-basic (pKa of MnO3(OH) = −2.25), it can be activated by AcOH or Ca2+ to oxidize cyclohexane at room temperature to give cyclohexanone as the major product. A synergistic effect occurs when both AcOH and Ca2+ are present; the relative rates for the oxidation of cyclohexane by MnO4−/AcOH, MnO4−/Ca2+ and MnO4−/AcOH/Ca2+ are 1 : 73 : 198. DFT calculations show that in the active intermediate of MnO4−/AcOH/Ca2+, MnO4− is H-bonded to 3 AcOH molecules, while Ca2+ is bonded to 3 AcOH molecules as well as to an oxo ligand of MnO4−. Our results also suggest that these synergistic activating effects of a weak Brønsted acid and a weak Lewis acid should be applicable to a variety of metal-oxo species.


 Please wait while we load your content...
Please wait while we load your content...