Expanding the hydride chemistry: antiperovskites A3MO4H (A = Rb, Cs; M = Mo, W) introducing the transition oxometalate hydrides†
Abstract
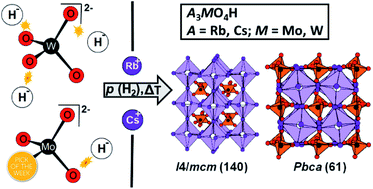
The four compounds A3MO4H (A = Rb, Cs; M = Mo, W) are introduced as the first members of the new material class of the transition oxometalate hydrides. The compounds are accessible via a thermal synthesis route with carefully controlled conditions. Their crystal structures were solved by neutron diffraction of the deuterated analogues. Rb3MoO4D, Cs3MoO4D and Cs3WO4D crystallize in the antiperovskite-like K3SO4F-structure type, while Rb3WO4D adopts a different orthorhombic structure. 2H MAS NMR, Raman spectroscopy and elemental analysis prove the abundance of hydride ions next to oxometalate ions and experimental findings are supported by quantum chemical calculations. The tetragonal phases are direct and wide band gap semiconductors arising from hydride states, whereas Rb3WO4H shows a unique, peculiar valence band structure dominated by hydride states.
- This article is part of the themed collections: 2022 ChemSci Pick of the Week Collection and 2022 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...