Solution structure of a thrombin binding aptamer complex with a non-planar platinum(ii) compound†
Abstract
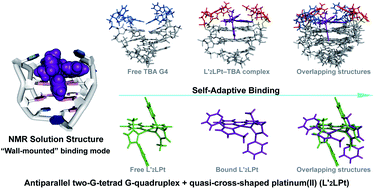
Thrombin Binding Aptamer (TBA) is a monomolecular well-defined two G-tetrad antiparallel G-quadruplex DNA that inhibits the activity of human α-thrombin. In this report, we synthesized a quasi-cross-shaped platinum(II) compound (L′2LPt) with one cyclometalated and two carbene ligands. We found L′2LPt has selective affinity to bind the TBA G-quadruplex. A fibrinogen clotting assay revealed that L′2LPt can abrogate the inhibitory activity of TBA against thrombin. We solved the 1 : 1 L′2LPt–TBA complex structure by NMR, which revealed a unique self-adaptive property of L′2LPt upon binding to TBA. In the complex, a carbene ligand of L′2LPt rotates to pair with the cyclometalated ligand to form a plane stacking over half of the TBA G-tetrad and covered by lateral TT loops. It is notable that the heavy atom Pt stays out of the G-tetrad. Meanwhile, the other carbene ligand remains relatively perpendicular and forms a hydrogen bond with a guanine to anchor the L′2LPt position. This structure exhibits a quasi-cross-shaped Pt(II) compound bound to the G-quadruplex with an unusual “wall-mounted” binding mode. Our structures provide insights into the specific recognition of antiparallel G-quadruplex DNA by a self-adaptive Pt(II) compound and useful information for the design of selective G-quadruplex targeting non-planar molecules.


 Please wait while we load your content...
Please wait while we load your content...