Robust dicopper(i) μ-boryl complexes supported by a dinucleating naphthyridine-based ligand†
Abstract
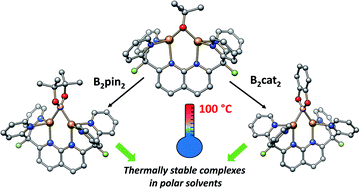
Copper boryl species have been widely invoked as reactive intermediates in Cu-catalysed C–H borylation reactions, but their isolation and study have been challenging. Use of the robust dinucleating ligand DPFN (2,7-bis(fluoro-di(2-pyridyl)methyl)-1,8-naphthyridine) allowed for the isolation of two very thermally stable dicopper(I) boryl complexes, [(DPFN)Cu2(μ-Bpin)][NTf2] (2) and [(DPFN)Cu2(μ-Bcat)][NTf2] (4) (pin = 2,3-dimethylbutane-2,3-diol; cat = benzene-1,2-diol). These complexes were prepared by cleavage of the corresponding diborane via reaction with the alkoxide [(DPFN)Cu2(μ-OtBu)][NTf2] (3). Reactivity studies illustrated the exceptional stability of these boryl complexes (thermal stability in solution up to 100 °C) and their role in the activation of C(sp)–H bonds. X-ray diffraction and computational studies provide a detailed description of the bonding and electronic structures in these complexes, and suggest that the dinucleating character of the naphthyridine-based ligand is largely responsible for their remarkable stability.


 Please wait while we load your content...
Please wait while we load your content...