Protecting group free glycosylation: one-pot stereocontrolled access to 1,2-trans glycosides and (1→6)-linked disaccharides of 2-acetamido sugars†
Abstract
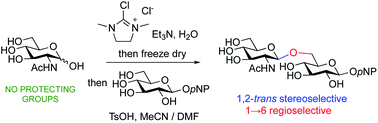
Unprotected 2-acetamido sugars may be directly converted into their oxazolines using 2-chloro-1,3-dimethylimidazolinium chloride (DMC), and a suitable base, in aqueous solution. Freeze drying and acid catalysed reaction with an alcohol as solvent produces the corresponding 1,2-trans-glycosides in good yield. Alternatively, dissolution in an aprotic solvent system and acidic activation in the presence of an excess of an unprotected glycoside as a glycosyl acceptor, results in the stereoselective formation of the corresponding 1,2-trans linked disaccharides without any protecting group manipulations. Reactions using aryl glycosides as acceptors are completely regioselective, producing only the (1→6)-linked disaccharides.


 Please wait while we load your content...
Please wait while we load your content...