A different polynorbornene backbone by combination of two polymer growth pathways: vinylic addition and ring opening via β-C elimination†
Abstract
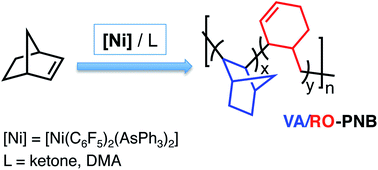
A new polynorbornene skeleton has been found that contains bicyclic norbornane units and cyclohexenyl methyl linkages. The polymers have been synthesized using a nickel catalyst in the presence of a controlled amount of ligands with low or moderate coordination ability. The backbone structure is the result of a vinylic addition polymerization, via sequential insertions of norbornene into a Ni–C bond (bicyclic units) combined with an unusual ring opening of the norbornene structure by a β-C elimination (cyclohexenyl methyl units) to give a new Ni–C(alkyl) bond that continues the polymerization. The ring opening events are favored when the rate of propagation of the vinylic addition polymerization decreases, and this can be modulated by making the coordination of norbornene to the metal center less favorable using additional ligands.


 Please wait while we load your content...
Please wait while we load your content...