C(sp3)–H oxygenation via alkoxypalladium(ii) species: an update for the mechanism†
Abstract
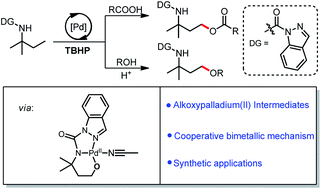
Pd-catalyzed C(sp3)–H oxygenation has emerged as an attractive strategy for organic synthesis. The most commonly proposed mechanism involves C(sp3)–H activation followed by oxidative addition of an oxygen electrophile to give an alkylpalladium(IV) species and further C(sp3)–O reductive elimination. In the present study of γ-C(sp3)–H acyloxylation of amine derivatives, we show a different mechanism when tert-butyl hydroperoxide (TBHP) is used as an oxidant—namely, a bimetallic oxidative addition-oxo-insertion process. This catalytic model results in an alkoxypalladium(II) intermediate from which acyloxylation and alkoxylation products are formed. Experimental and computational studies, including isolation of the putative post-oxo-insertion alkoxypalladium(II) intermediates, support this mechanistic model. Density functional theory reveals that the classical alkylpalladium(IV) oxidative addition pathway is higher in energy than the bimetallic oxo-insertion pathway. Further kinetic studies revealed second-order dependence on [Pd] and first-order on [TBHP], which is consistent with DFT analysis. This procedure is compatible with a wide range of acids and alcohols for γ-C(sp3)–H oxygenation. Preliminary functional group transformations of the products underscore the great potential of this protocol for structural manipulation.


 Please wait while we load your content...
Please wait while we load your content...