Highly effective and chemoselective hydrodeoxygenation of aromatic alcohols†
Abstract
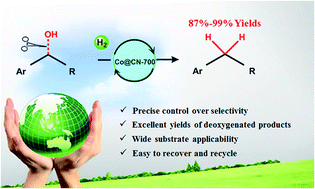
Effective hydrodeoxygenation (HDO) of aromatic alcohols is very attractive in both conventional organic synthesis and upgrading of biomass-derived molecules, but the selectivity of this reaction is usually low because of the competitive hydrogenation of the unsaturated aromatic ring and the hydroxyl group. The high activity of noble metal-based catalysts often leads to undesired side reactions (e.g., saturation of the aromatic ring) and excessive hydrogen consumption. Non-noble metal-based catalysts suffer from unsatisfied activity and selectivity and often require harsh reaction conditions. Herein, for the first time, we report chemoselective HDO of various aromatic alcohols with excellent selectivity, using porous carbon–nitrogen hybrid material-supported Co catalysts. The C–OH bonds were selectively cleaved while leaving the aromatic moiety intact, and in most cases the yields of targeted compounds reached above 99% and the catalyst could be readily recycled. Nitrogen doping on the carbon skeleton of the catalyst support (C–N matrix) significantly improved the yield of the targeted product. The presence of large pores and a high surface area also improved the catalyst efficiency. This work opens the way for efficient and selective HDO reactions of aromatic alcohols using non-noble metal catalysts.


 Please wait while we load your content...
Please wait while we load your content...