Ir/f-Ampha complex catalyzed asymmetric sequential hydrogenation of enones: a general access to chiral alcohols with two contiguous chiral centers†
Abstract
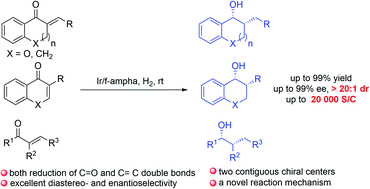
A general and highly efficient method for asymmetric sequential hydrogenation of α,β-unsaturated ketones has been developed by using an iridium/f-Ampha complex as the catalyst, furnishing corresponding chiral alcohols with two contiguous stereocenters in high yields with excellent diastereo- and enantioselectivities (up to 99% yield, >20 : 1 dr and >99% ee). Control experiments indicated that the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and C
C and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds of the enones were hydrogenated sequentially, and the final stereoselectivities were determined by the dynamic kinetic resolution of ketones. Moreover, DFT calculations revealed that an outer sphere pathway was involved in both reduction of C
O bonds of the enones were hydrogenated sequentially, and the final stereoselectivities were determined by the dynamic kinetic resolution of ketones. Moreover, DFT calculations revealed that an outer sphere pathway was involved in both reduction of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and C
C and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds of enones. The synthetic utility of this method was demonstrated by a gram-scale reaction with very low catalyst loading (S/C = 20 000) and a concise synthetic route to key chiral intermediates of the antiasthmatic drug CP-199,330.
O bonds of enones. The synthetic utility of this method was demonstrated by a gram-scale reaction with very low catalyst loading (S/C = 20 000) and a concise synthetic route to key chiral intermediates of the antiasthmatic drug CP-199,330.


 Please wait while we load your content...
Please wait while we load your content...