The transesterification of ethylene glycol and 1,2-butanediol with dimethyl carbonate: reaction network and kinetic modeling†
Abstract
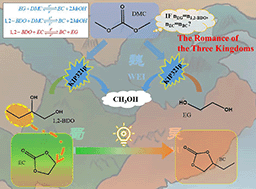
Reactive distillation is an effective technique for separating azeotropic systems, especially ethylene glycol (EG) and 1,2-butanediol (1,2-BDO), and mastering the reaction network and competitive kinetics is vital for the successful design of a reactive distillation process. Herein, a reactive distillation process using dimethyl carbonate (DMC) as a reactant to recover EG and the high-value-added byproduct 1,2-BC was developed. The reaction systems EG–DMC, 1,2-BDO–DMC, and EC–1,2-BDO were investigated through experiments in the presence of the catalyst KIP321p and the kinetic parameters were subjected to regression analysis; a unified power-law model describes the kinetics of transesterification reactions very well. The heat absorption values of the EG–DMC and 1,2-BDO–DMC systems are 7.18 kJ mol−1 and 9.56 kJ mol−1, respectively; as EG has fewer ethyl groups in its molecular structure than 1,2-BDO, this lower spatial resistance resulted in a faster reaction rate under the same conditions. The enthalpy change of the EC–1,2-BDO system is 1.53 kJ mol−1, and the small positive value indicates that BC is more thermodynamically stable than EC. The transesterification of EG/1,2-BDO with DMC was also investigated experimentally, and the experimental data were compared with the predicted results from the reaction kinetic models obtained in this work. These kinetic parameters can be employed to design reactive distillation methods for the implementation of transesterification reactions of mixed glycols with DMC.


 Please wait while we load your content...
Please wait while we load your content...