A continuous flow investigation of sulfonyl chloride synthesis using N-chloroamides: optimization, kinetics and mechanism†
Abstract
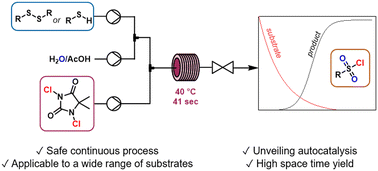
Sulfonyl chlorides are essential key building blocks in organic chemistry, especially for the preparation of sulfonamide motifs. However, conventional methods for the preparation of sulfonyl chlorides typically use difficult-to-handle reagents and the reactions are highly exothermic. In this work, we develop a continuous flow protocol for the synthesis of sulfonyl chlorides from disulfides and thiols, using 1,3-dichloro-5,5-dimethylhydantoin (DCH) as a dual-function reagent for oxidative chlorination. Temporal kinetic profiles were collected which indicated unusual sigmoidal behavior for product formation. We also identified several intermediates, which provided an insight into the reaction mechanism. A small reactor volume (639 μL) and short residence time (41 s) afforded a very high space–time yield (6.7 kg L−1 h−1) for the model system. The optimal conditions were applied to react aliphatic and aromatic disulfides and thiols to their corresponding sulfonyl chlorides in good yields. The successful operation within a continuous flow environment enabled exquisite control over the reaction parameters, and improved the inherent safety of the process by circumventing thermal runaway.


 Please wait while we load your content...
Please wait while we load your content...