Experimental insights into catalytic oxidation of 1,6-hexanediol to ε-caprolactone over (p-cymene)RuCl2(L) complexes in non-polar media†
Abstract
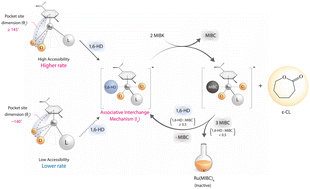
1,6-Hexanediol (1,6-HD) oxidation to ε-caprolactone (ε-CL) was investigated using (p-cymene)RuCl2(L) complexes with phosphine (LP1–P5) and pyridine (LN1–N5) ligands as catalysts. Despite similar Ru electron density, the activity of (p-cymene)RuCl2(LP) increases with the decrease in phosphine steric hindrance rather than the electronic properties. The reaction rate correlates with the pocket-size dimension (θc), defined by the (centroid of the p-cymene ring)-Ru-(centroid of 2Cl) angle of the (p-cymene)RuCl2(L) complexes. This observation supports an associative interchange mechanism previously proposed by computational studies. The readily accessible θc (≥145°) of these LN complexes results in a similar rate, regardless of different LN ligands. For both complexes, the ε-CL selectivity depends only on 1,6-HD conversion. Even though bases significantly enhance the activity, they readily affect the complex stability. The methyl isobutyl carbinol (MIBC) produced during the reaction could competitively react with the Ru catalysts, leading to catalyst deactivation, especially when MIBC : 1,6-HD ≥ 2 times.


 Please wait while we load your content...
Please wait while we load your content...