DIPEA-induced activation of OH− for the synthesis of amides via photocatalysis†
Abstract
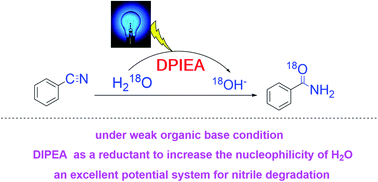
The development of green protocols for photocatalysis where water acts as a nucleophile, induced by a weak organic base, is difficult to achieve in organic chemistry. Herein, an efficient light-mediated strategy for the synthesis of amides in which a weak organic base acts as a reductant to induce the formation of OH– from water under metal-free conditions is reported. A mechanistic study reveals that the generation of an N,N-diisopropylethylamine (DIPEA) radical via single electron transfer (SET), with the assistance of photocatalyst, that increases the nucleophilicity of the water molecules with respect to the cyanides is essential. Moreover, the removal rate of nitrile in wastewater can be as high as 83%, indicating that this strategy has excellent potential for nitrile degradation.


 Please wait while we load your content...
Please wait while we load your content...