Protonation-induced charge transfer and polaron formation in organic semiconductors doped by Lewis acids
Abstract
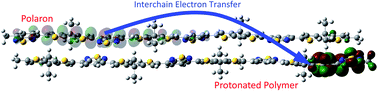
Lewis-acid doping of organic semiconductors (OSCs) opens up new ways of p-type doping and has recently become of significant interest. As for the mechanistic understanding, it was recently proposed that upon protonation of the OSC backbone, electron transfer occurs between the protonated polymer chain and a neutral chain nearby, inducing a positive charge carrier in the latter [B. Yurash, D. X. Cao, V. Brus et al., Nat. Mater., 2019, 18, 1327–1334]. To further clarify the underlying microscopic processes on a molecular level, in the present work, we theoretically analyze the influence of protons on the electronic properties of the widely used PCPDT-BT copolymer as a typical example. While we find that single protonation leads to formation of a localized polaron, double protonation leads to the release of a more delocalized polaron via an intrachain electron transfer. We also demonstrate the possibility of an interchain electron transfer. The vertical excitation spectra simulated for an ensemble of protonated polymers with different amounts of protons enable a detailed interpretation of the experimental observations and contribute to a molecular-level interpretation of the Lewis-acid doping process.


 Please wait while we load your content...
Please wait while we load your content...