Micellization and thermodynamics study of ester functionalized picoline-based ionic liquid surfactants in water†
Abstract
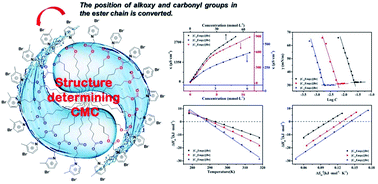
A novel series of picoline-based ionic liquid surfactants, N-alkyoxycarbonyl-3-picoline bromides [CnEmpy][Br] (n = 10, 12, 14), have been synthesized. The thermal stability, aggregation behavior and surface activity of the synthetic ionic liquid surfactants were investigated systematically though a series of methods, such as thermogravimetric analysis (TGA), differential scanning calorimetry (DSC), tensiometry and conductivity. The thermodynamics of micellization of the ionic liquid surfactants solution were studied by using the conductivity method in the temperature range 278.15–318.15 K. The surface activity parameters and thermodynamics parameters were derived, respectively. The enthalpy–entropy compensation effect was further discussed by using relative thermodynamics parameters. It was found that the [CnEmpy][Br] have moderate surface activity, and their critical micelle concentration (CMC) decreased with the ester-functionalized chain length and exhibited a U-shape with temperature. The calculation results of the thermodynamic parameters showed that the micellization processes of [CnEmpy][Br] were spontaneous, endothermic at low temperature and exothermic at higher temperature.


 Please wait while we load your content...
Please wait while we load your content...