5-N-Arylaminothiazoles with pyridyl groups and their first-row transition metal complexes: synthesis, photophysical properties, and Zn sensing†
Abstract
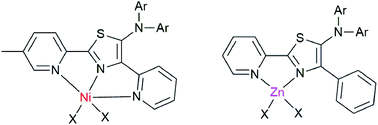
A series of 5-N-arylaminothiazoles were synthesized with facile diversity-oriented synthesis from readily available starting materials via the reaction of thioamide dianions and thioformamides. The introduction of various substituents at the 2-position of a thiazole ring (i.e., 2-pyridyl, 4-methylpyridyl, and phenyl groups) and on the nitrogen atom (i.e., p-tolyl and phenyl groups) significantly influenced the absorption and emission spectra of the isolated compounds. X-ray analysis confirmed that the substituents at the amino site were twisted from a thiazole ring, while the formation of its nickel complex showed dinuclear metal complexes bridged with chlorine atoms. Moreover, the formation of zinc–thiazole complexes showed enhanced emission properties in solution and noticeable emission in a solid state. In addition, thiazole-bridged dypyrromethene type ligands showed high selectivity toward Zn+2, which make them good candidates for zinc sensing.


 Please wait while we load your content...
Please wait while we load your content...