Electrochemical reduction of NO catalyzed by boron-doped C60 fullerene: a first-principles study†
Abstract
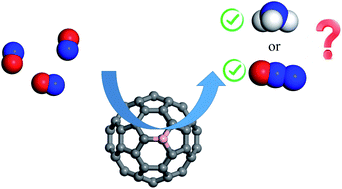
The electrochemical reduction of nitrogen monoxide (NO) is one of the most promising approaches for converting this harmful gas into useful chemicals. Using density functional theory calculations, the work examines the potential of a single B atom doped C60 fullerene (C59B) for catalytic reduction of NO molecules. The results demonstrate that the NO may be strongly activated over the B atom of C59B, and that the subsequent reduction process can result in the formation of NH3 and N2O molecules at low and high coverages, respectively. Based on the Gibbs free energy diagram, it is inferred that the C59B has excellent catalytic activity for NO reduction at ambient conditions with no potential-limiting. At normal temperature, the efficient interaction between the *NOH and NO species might lead to the spontaneous formation of the N2O molecule. Thus, the findings of this study provide new insights into NO electrochemical reduction on heteroatom doped fullerenes, as well as a unique strategy for fabricating low-cost NO reduction electrocatalysts with high efficiency.


 Please wait while we load your content...
Please wait while we load your content...