Acid-catalyzed ring-expansion of 4-(1-hydroxycyclobutyl)-1,2,3-triazoles†
Abstract
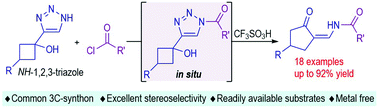
We here report a direct ring-opening/semipinacol rearrangement reaction of 4-(1-hydroxycyclobutyl)-1,2,3-triazole, in which N-acyl substituted 1,2,3-triazole was generated in situ and would trigger thermodynamically controlled electrocyclization ring-opening to afford a rearrangement precursor (α-diazo-ol). This strategy avoided the usage of metal catalysts and the reservation of sulfonyl groups on the N1-position of 1,2,3-triazole. The final cycloenaminone product is highly reactive and could be commonly used as a dinucleophilic acceptor to synthesize structurally diverse fused bicyclic products.


 Please wait while we load your content...
Please wait while we load your content...