Carbene-enabled ether activation through the formation of oxonium: a theoretical view†
Abstract
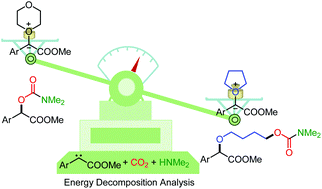
Here, we report a theoretical investigation of the reactivity and chemoselectivity of carbene-enabled ether activation. The mechanism obtained from DFT calculations revealed that the final products were dependent on the stability of the oxonium intermediate, which was afforded by nucleophilic attack of the corresponding ether onto a carbene species. Interestingly, we found that the nucleophilicity of ethers was crucial but not their solvation. Energy decomposition analysis based on absolutely localized molecular orbitals showed that the nucleophilicity of ethers was critical for polarization and charge transfer, leading to the chemoselectivity.


 Please wait while we load your content...
Please wait while we load your content...