Photoinduced C–H monofluoroalkenylation with gem-difluoroalkenes through hydrogen atom transfer under batch and flow conditions†
Abstract
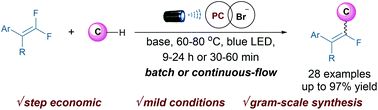
The direct monofluoroalkenylation of C–H bonds is a straightforward approach for accessing multisubstituted monofluoroalkenes. However, general methods for the monofluoroalkenylation of C(sp3)–H bonds remain challenging. Here we report the development of a mild monofluoroalkenylation of C–H bonds with gem-difluoroalkenes through the synergetic merger of photoredox and bromine-based hydrogen atom transfer catalysis. This strategy uses a wide range of C–H patterns, including ethers, amides and aliphatic aldehydes, to afford multisubstituted monofluoroalkenes in an effective manner. The reaction can be rapidly accelerated in a continuous-flow protocol, which was amenable to gram scale synthesis. Furthermore, the merit of this method was demonstrated by the ability to rapidly access several valuable and complex molecules from readily available C–H feedstocks in a highly selective fashion. Preliminary mechanistic experiments reveal that the reaction proceeded through a radical–radical cross-coupling between alkyl radicals and monofluoroalkenyl radicals.


 Please wait while we load your content...
Please wait while we load your content...