Palladium-catalyzed tandem Heck/carbonylation/aminocarbonylation en route to chiral heterocyclic α-ketoamides†
Abstract
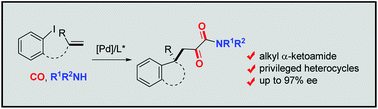
An unprecedented tandem carbonylation/aminocarbonylation triggered by palladium-catalyzed enantioselective Heck-type exocyclopalladation delivering chiral heterocyclic α-ketoamides has been developed. The uncommon double CO insertion into the σ-alkylpalladium intermediate takes place selectively under atmospheric pressure of CO using alkylamine as the nucleophile. Unique structures hybridizing alkyl α-ketoamides with indolin-2-one containing a C3 all-carbon quaternary stereogenic center and 5,7-dihydro-6H-dibenzo[b,d]azepin-6-one containing a thermodynamically stabilized stereogenic biaryl axis are produced in good yields with excellent enantio- and/or diastereoselectivities.


 Please wait while we load your content...
Please wait while we load your content...