Regio- and stereo-selective olefinic C–H functionalization of aryl alkenes in ethanol†
Abstract
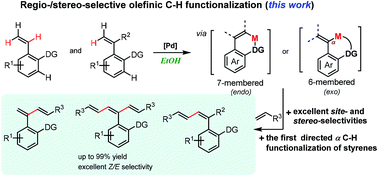
We report on N,N-bidentate-chelation-assisted α- and β-olefinic C–H alkenylation of aryl alkenes in ethanol to afford aryl dienes/trienes with excellent regio- and stereo-selectivities. The reaction of 2-alkenyl benzylamine and benzoic acid derived substrates proceeded through six-membered exo-cyclometallation and seven-membered endo-cyclometallation. The aerobic protocols feature wide functionality tolerance, high selectivities and yields, mild conditions and scalable preparation, and the directing group can be easily removed to afford Boc-protected amine by simple reduction.


 Please wait while we load your content...
Please wait while we load your content...