Eugenunilones A–H: rearranged sesquiterpenoids from Eugenia uniflora†
Abstract
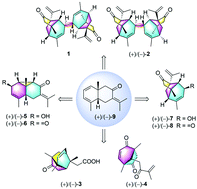
Six rearranged sesquiterpenoid dimers and monomers with four types of new skeletons, meso-eugenunilone A (meso-1), (+)- and (−)-eugenunilones B−F [(+)- and (−)-2–6], along with two new biogenetically related members (+)- and (−)-eugenunilones G−H [(+)- and (−)-7 and 8] and a known one (9), were isolated from the fruits of Eugenia uniflora. Compounds 1 and 2 were two unprecedented dimers with caged tricyclo[4.4.0.02,8]decane units. Compounds 3 and 4 represent the first examples of sesquiterpenoids possessing a caged tricyclo[4.3.1.03,7]decane core and an isopentyl substituted bicyclo[3.2.1]octane backbone, respectively, while compounds 5 and 6 share a tricyclo[4.4.0.02,10]decane scaffold which is found in nature for the first time. In particular, compounds 2–9 were successfully separated into eight pairs of optically pure enantiomers by chiral HPLC separation. Their structures and absolute configurations were unambiguously established by a combination of spectroscopic data, X-ray diffraction analysis, and electronic circular dichroism (ECD) calculations. A hypothetical biogenetic pathway for these new compounds was proposed. It is interesting that these diverse frameworks were all derived from the same bicyclic precursor (9). In addition, compounds 2, 4, and 6 showed moderate anti-inflammatory effects in zebrafish acute inflammatory models.


 Please wait while we load your content...
Please wait while we load your content...