A Ni(ii)-catalyzed reductive cross-coupling reaction of oxalates and thiosulfonates/selenosulfonates†
Abstract
A Ni(II)-catalyzed reductive cross-coupling reaction of oxalates and thiosulfonates/selenosulfonates to synthesize benzylic sulfides/selenides under mild conditions is developed. The oxalates prepared from the corresponding alcohols are used as carbon radical precursors to participate in the reaction. This strategy has the advantages of easy substrate availability and mild conditions, and provides a new method for the preparation of unsymmetrical sulfides/selenides with good functional group tolerance.
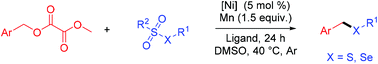


 Please wait while we load your content...
Please wait while we load your content...