Palladium-catalyzed tandem hydrocarbonylative cycloaddition for expedient construction of bridged lactones†
Abstract
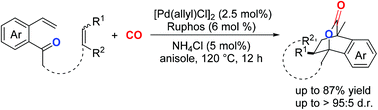
A palladium-catalyzed tandem carbonylative lactonization and cycloaddition reaction of 2-vinyl acetophenones with alkenes and CO has been established. This reaction enables an efficient conversion of the easily available alkenes to various bridged lactones through intermolecular cycloaddition. Furthermore, an intramolecular tandem hydrocarbonylative lactonization and cycloaddition reaction could also be established to access more complex bridged lactones by this strategy.


 Please wait while we load your content...
Please wait while we load your content...