In situ phosphonium-containing Lewis base-catalyzed 1,6-cyanation reaction: a facile way to obtain α-diaryl and α-triaryl acetonitriles†
Abstract
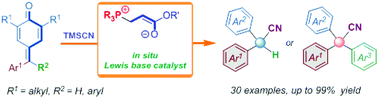
We present a phosphonium-containing catalyst generated in situ from phosphine and tert-butyl acrylate that serves as an unusual Lewis base catalyst. It was applied for the promotion of a remote 1,6-cyanation reaction of p-quinone methides and fuchsones employing trimethylsilyl cyanide as the cyanide source. A diverse range of α-diaryl and α-triaryl acetonitriles was obtained in high yields under mild reaction conditions with low catalyst loading (5 mol%). The practicality and utility of this protocol were demonstrated via the gram-scale preparation and facile elaboration of products. Mechanistic investigations (in situ NMR and ESI-MS analysis) were employed to characterize the active zwitterionic phosphonium intermediate, which was the “true” active catalyst.


 Please wait while we load your content...
Please wait while we load your content...