Electrochemical synthesis of functionalized gem-difluoroalkenes with diverse alkyl sources via a defluorinative alkylation process†
Abstract
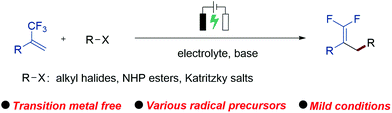
An electrochemical defluorinative alkylation protocol of α-trifluoromethyl alkenes is described. This reaction enables the preparation of functionalized gem-difluoroalkenes with the use of diverse alkyl sources including organohalides, N-hydroxyphthalimide (NHP) esters and Katritzky salts. This method exhibits lots of synthetic advantages including mild conditions, simple operation, and convenience of amplification, and provides a new route for the synthesis of gem-difluoroalkenes.


 Please wait while we load your content...
Please wait while we load your content...