Solvent-controlled regioselective arylation of indoles and mechanistic explorations†
Abstract
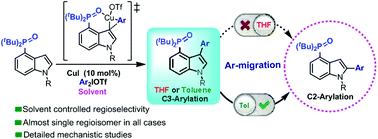
The ability to manipulate the site selectivity in C–H bond functionalization reactions is an important goal in modern chemistry. Herein, we report a new reaction strategy for the regioselective arylation of indoles at C2 or C3 positions, achieved by adjusting the solvent and with P(O)tBu2 as an auxiliary group. The experimental results and density functional theory (DFT) calculations confirmed that Cu(III) dominated the formation of C3-arylation products, and the acid produced in this process initiated the migration of the C3-aryl group to the C2 site. THF, with its coordination ability, could bind acid and inhibit the migration, while the domino process in toluene proceeded smoothly. Based on this methodology, some classic indole-based fluorescent molecules synthesized by us exhibit substantially better optical properties due to the intervention of P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O functional groups.
O functional groups.


 Please wait while we load your content...
Please wait while we load your content...