Palladium-catalyzed direct construction of oxazoline-containing polycyclic scaffolds via tandem addition/cyclization of nitriles and arylboronic acids†
Abstract
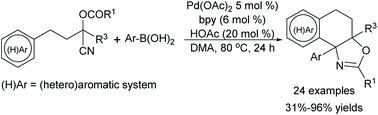
An efficient construction of oxazoline-containing polycyclic scaffolds is presented through a Pd-catalyzed addition/cyclization of (hetero)arene tethered O-acyl cyanohydrin units and arylboronic acids. Diverse oxazoline-containing polycyclic compounds can be prepared in good to high yields under mild reaction conditions. This method can be extended to prepare multi-substituted polycyclic heterocyclic compounds via a Pd-catalyzed addition/cyclization/arylation tandem sequence using arylboronic acid and different heteroarene scaffolds such as thiophene, pyrrole and furan analogues.


 Please wait while we load your content...
Please wait while we load your content...